Background: Covalent Bruton's tyrosine kinase inhibitors (cBTKis) and venetoclax (Ven) constitute standard-of-care treatment for patients (pts) with CLL/SLL. As more pts are treated with these agents, the population exposed to both agents (double exposed) grows, as does the cohort regarded as double refractory (progressed on both classes). Pts who are therapy intolerant and those with double-refractory disease need alternative treatment options. This study aims to evaluate outcomes among double-exposed and double-refractory pts.
Methods: In this single-institution, retrospective study, we used our institutional CLL database to identify pts with a CLL/SLL diagnosis who were previously treated with both Ven and a cBTKi. Patient-level clinical data were extracted from electronic medical records. Progressive disease (PD) was defined by the treating physician based on clinical, laboratory, and/or imaging data. Progression-free survival (PFS) and Overall survival (OS) were calculated using the Kaplan-Maier estimates from the time pts required next-line treatment for PFS and from the time pts concluded therapy with both classes for the OS.
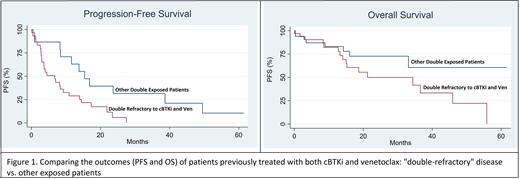
Results: We identified 67 pts who required any treatment for CLL/SLL between 2001 and 2020 and received both cBTKi and Ven. The median age was 69 (range: 50-84), and 64% were male. FISH abnormalities included 24% del17p, 13% del11q, 24% del 13q, and 15% with trisomy 12. Pts received a median of 6 lines of therapy (range: 2-12). The most common cBTKi was ibrutinib (96%), followed by acalabrutinib (22%) and zanubrutinib (3%). A total of 12 pts (18%) received more than one prior cBTKi. Pts received a median of 22.4 months of a cBTKi, and a median of 11 months of Ven. Only 6 pts (9%) were intolerant to both a cBTKi and Ven, whereas 17 (25%) were intolerant to a cBTKi alone, and 6 (9%) were intolerant to Ven alone. Thirty-two (48%) pts were considered double refractory after experiencing progressive disease on both Ven and a cBTKi. Pts received a median of 1 treatment line after exposure to both Ven and a cBTKi (range: 0-4). The most common immediate treatments were CAR-T (22; 47%), PI3K inhibitor (4; 9%), and non-covalent BTKi (3; 6%). The median OS was 36.6 months in the entire double-exposed cohort (n=66; 95% CI: 19-53). For double refractory pts, the median OS was 21.2 months (n = 32; 95% CI: 14-46), whereas the median OS was not reached for pts who were double exposed but not double refractory (n=34; logrank p-value =0.004; Fig. 1). The median PFS was 8.4 months (95% CI: 4-14) for the 46 double-exposed pts who started another treatment line. Double refractory pts had a median PFS of only 6.8 months (n=30; 95% CI: 3-9), unlike pts who were not double refractory and had a median PFS of 15.4 months (n-16; 95% CI: 8-39; logrank p-value = 0.01; Fig. 1).
Conclusion: We provide clinical benchmarks for CLL/SLL pts after treatment with both a cBTKi and Venetoclax. Pts with double refractory disease had a significantly shorter PFS and OS compared to others. These data are particularly relevant to clinical practice as they encourage participation in clinical trials and inform the design of trials investigating novel agents in this space. Drug development for pts previously treated with cBTKi and Ven remains an unmet need.
Disclosures
Ujjani:Kite, a Gilead Company: Consultancy, Other: Travel expenses , Research Funding; PCYC: Research Funding; Atara: Consultancy; Astrazeneca: Consultancy, Honoraria, Research Funding; Epizyme: Consultancy; Lilly: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria, Research Funding. Lynch:Cancer Study Group: Consultancy; Rapt: Research Funding; SeaGen: Research Funding; Genentech: Research Funding; Cyteir: Research Funding; Bayer: Research Funding; Incyte: Research Funding; TG Therapeutics: Research Funding; SeaGen: Consultancy; Foresight Diagnostics: Consultancy; Abbvie: Consultancy; Merck: Research Funding. Poh:Seattle Genetics: Consultancy; BeiGene: Consultancy; Incyte: Research Funding; Acrotech: Consultancy. Smith:ADC Therapeutics, AstraZeneca, Ayala (spouse), Bayer, BeiGene, Bristol Myers Squibb (spouse), De Novo Biopharma, Enterome, Genentech, Inc., Ignyta (spouse), Incyte Corporation, Kymera Therapeutics, Merck Sharp and Dohme Corp., MorphoSys, Nanjing Pharmaceu: Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics, AstraZeneca, BeiGene, Epizyme, Karyopharm, KITE pharma, Incyte, Numab Therapeutics AG, Abbvie, Coherus Biosciences, advisory board (spouse) Genentech, Inc.: Consultancy. Till:BMS/Juno Therapeutics: Research Funding; Mustang Bio: Consultancy, Patents & Royalties, Research Funding; Proteios Technology: Consultancy, Current holder of stock options in a privately-held company. Maloney:Novartis: Consultancy, Honoraria; Mustang Bio: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; A2 Biotherapeutics: Consultancy, Current holder of stock options in a privately-held company, Honoraria, Other: Member of the Scientific Advisory Board; Juno Therapeutics: Consultancy, Honoraria, Patents & Royalties: Rights to royalties from Fred Hutch for patents licensed to Juno Therapeutics/BMS, Research Funding; Kite, a Gilead Sciences: Consultancy, Honoraria, Research Funding; Legend Biotech: Consultancy, Honoraria, Research Funding; Pharmacyclics: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria, Other: Member, Scientific Review Committee, Research Scholars Program in Hematologic Malignancies; Incyte: Consultancy, Honoraria; Genentech: Consultancy, Honoraria, Other: Chair and Member of the Lymphoma Steering Committee; Janssen: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Participation on a Data Safety Monitory Board , Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Other: Member of the JCAR017 EAP-001 Safety Review Committee and Member, CLL Strategic Council, Member of the JCAR017-BCM-03 Scientific Steering Committee under BMS, Research Funding; Umoja: Consultancy, Honoraria; Navan Technologies: Consultancy, Honoraria, Other: Member of the Scientific Advisory Board; Bioline Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Participation on a Data Safety Monitory Board ; Fred Hutch: Other: rights to royalties for patents licensed to Juno; Navan Technologies: Current holder of stock options in a privately-held company; Chimeric Therapeutics: Other: Member of the Scientific Advisory Board; ImmPACT Bio: Other: Member, Clinical Advisory Board, CD19/CD20 bi-specific CAR-T Cell Therapy Program; Interius: Other: Member, Clinical Advisory Board; Lyell Immunopharma: Other: Member, CAR T Steering Committee. Gopal:Compliment Corporation: Current holder of stock options in a privately-held company; Merck, I-Mab bio, IgM Bio, Takeda, Gilead, Astra-Zeneca, Agios, Janssen, BMS, SeaGen, Teva, Genmab: Research Funding; Incyte, Kite, Morphosys/Incyte, ADCT, Acrotech, Merck, Karyopharm, Servier, Beigene, Cellectar, Janssen, SeaGen, Epizyme, I-Mab bio, Gilead, Genentech, Lilly, Caribou, Fresenius-Kabi: Consultancy. Shadman:Genentech: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Genmab: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Eli Lilly: Consultancy; Janssen: Consultancy; AstraZeneca: Consultancy, Research Funding; MEI Pharma: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Mustang Bio: Consultancy, Research Funding; Fate Therapeutics: Consultancy; ADC therapeutics: Consultancy; MorphoSys/Incyte: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy; Vincerx: Research Funding; Regeneron: Consultancy; TG Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal